top of page
Insights & News


The Patent Sweet Spot: How to Identify the Real Value in a Biotech Patent Family
After more than 25 years working with biotech innovators, I’ve learned that the real value of a patent family lies in the inventive scope where your science, legal strategy, and innovation truly meet . That intersection is what I call the patent sweet spot . So, what exactly is the sweet spot? It’s the range of claim scope that matches the working examples in your application — broad enough to protect what matters commercially, but grounded enough in data to avoid overreach
Oct 15, 2025


Building Lasting Innovation: Lessons from My Decade+ at Invitrogen
Innovation, collaboration, and thoughtful IP strategy, that’s what built Invitrogen’s success. And it’s still what drives the best biotech companies today. In 2004, I joined Invitrogen , one of the most dynamic biotech companies of its time. Recently, I came across my old Invitrogen catalog from that year, a snapshot of the start of the company’s golden era, and it brought back a flood of memories. The catalog reads like a roll call of transformative tools: Lipofectamine, Su
Oct 9, 2025


Is Your Biotech Company Dedicated to Strong Patents?
The Lip Service Problem In 25+ years of working with biotech leadership teams, I’ve heard countless executives assure investors, employees, and boards: “We are committed to strong patents.” But too often, those words are just lip service. Even well-meaning leadership teams that believe they are prioritizing IP often aren’t. The difference between talk and true commitment comes down to one rarely asked question: “What percentage of your R&D budget is dedicated to experiments r
Sep 24, 2025


Strong Patents: Lip Service vs. True Commitment
Every biotech CEO says it. “We are committed to strong patents.” But here’s the uncomfortable truth: words don’t build patent fortresses. Budgets do. The question that separates talk from true commitment is simple: 👉 What percentage of your R&D budget is dedicated to experiments requested by your patent team to strengthen and broaden patents? If the answer is $0 , or “we don’t really track that,” then your company may be more vulnerable than you realize. Without patent-stre
Sep 24, 2025


AI Hype vs. Reality: Lessons from PCs, the Internet, and Smartphones
As a professional whose career started in the 1980s, AI feels like the early days of PCs, the Internet, and smartphones—big hype, real potential, but real transformation takes time. Even if the tech moves fast, humans and systems take longer to adapt. ---------------- [FYI: The above text was generated by ChatGPT except that I added the 1st clause: "As a professional whose career started in the 1980s". (I left in the em dash (—) so you might have picked up that this was gener
Aug 20, 2025


Afraid that AI might take your job?
At DHL, we are committed to finding ways to use AI to help us do our patent tasks more efficiently and more effectively. We use AI every day to help us. But we realize that AI is in its early days and has many limitations. That's why we use it carefully and responsibly. About DHL Double Helix Law (DHL) has decades of experience building strong patent portfolios for life science companies. Learn more about DHL and meet the team . Want to stay updated on life science patent law
Aug 19, 2025


Trouble Getting Biotech Patents to Issue? Don't Blame the Examiner.
Why Your Biotech Patent Keeps Getting Rejected, and What to Do About It. If your biotech patent application keeps getting rejected, it’s tempting to blame the patent examiner. But in most cases, the real issue is a weak patenting strategy or poor execution — both of which can be fixed. Here’s how to diagnose what’s going wrong and turn things around. Point 1 – Are You Relying on Only One Examiner and One Claiming Strategy? If you have only one application, one examiner, and
Aug 10, 2025


H1 2025 Bio/Pharma Funding Trends Report
“Bio/pharma funding deal data in H1 2025 reveals a troubling downward shift starting in February.” Investment in bio/pharma was down in H1 2025 according to data from the BiopharmIQ database¹. From the total first half data, this was most apparent in data related to public companies. However, more troubling were the downward trends in bio/pharma investment seen as 2025 progressed. Q2 2025 had the lowest private and post-IPO public funding activity of any quarter since Q1 2024
Jul 24, 2025

4 TIPS TO GET DIFFICULT BIOTECH PATENTS TO ISSUE
Struggling to get tough biotech patents allowed? A reactive, traditional "strategy" often fails. Try these 4 proactive, effective patenting tips to get valuable patents to issue. FILE A ROBUST PATENT APPLICATION It starts at the top; Give yourself your best chance at success with a robust patent application filing with numerous fallbacks supported by working examples with surprising results. FILE A DECLARATION DURING PROSECUTION WITH SUPPORTIVE AND SURPRISING DATA Strengthe
Jul 9, 2025

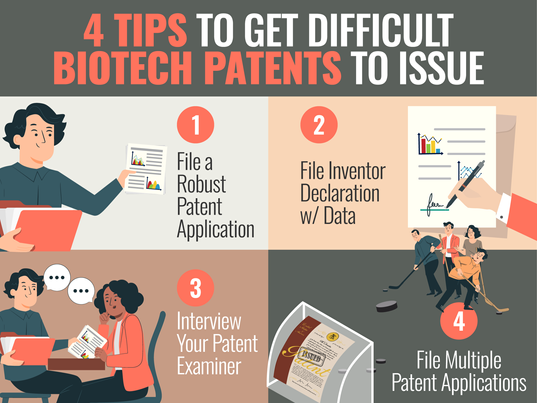
4 TIPS TO GET DIFFICULT BIOTECH PATENTS TO ISSUE IN THE U.S.
(Check out the short version of this post, if you don't want all the details in this patent professional version.) Introduction: It can often be difficult to get valuable patents covering biotech inventions to issue in the U.S. This is especially true if your patent team files quick and minimal patent applications and Office Action Responses, and uses a traditional reactive patent prosecution “strategy”. Alternatively, in this post, we provide 4 proactive and effective paten
Jul 8, 2025


KEEP YOUR ANNUAL IP SPEND WITHIN BUDGET WITH OUR PRIORITIZATION MATRIX TOOL (Full Version)
SUMMARY In this post we present a prioritization matrix tool that enables your IP team to dedicate an appropriate amount of resources to its highest value tasks and remain within its monthly, quarterly and annual budgets. For patent prosecution tasks, the matrix tool relies on prioritization not only at the patent family level, but at the individual patent application/country level as well. Such prioritization requires open and frequent communication with business, R&D, and
Jun 30, 2025


Assuring Your Patent And R&D Teams Are Well Connected
Biotech products and processes evolve over time. This can cause a misalignment between your issued/pending patent claims, and your improved processes and products. Thus, your patent team needs to stay closely connected to your R&D and product teams. Are your patent, product and R&D teams well connected? To find out, pose this question to your in-house IP coordinator/Chief patent counsel: Has an experienced patent attorney confirmed that the claims of our issued patents/pendin
May 7, 2025


Having Trouble Getting a Biotech Patent to Issue? Expert Declarations Can Clear the Way.
Biotech innovation is regularly faced with a thicket of prior art that applicants must navigate during patent prosecution. Examiners at the U.S. Patent and Trademark Office (USPTO) often combine various pieces of prior art to reject the claims as obvious. As we discuss in our recent article , arguments that can be made to overcome obviousness rejections, focus on the following with respect to the cited prior art: all the elements of the claims are not disclosed; a lack of a
Apr 20, 2025


Double Helix Law Turns 10!
This week, we’re celebrating a decade of helping life sciences innovators protect their groundbreaking IP. We are grateful for every client and team member who’s been part of this journey. To Our Clients Thank you for trusting us with your breakthrough technologies. Your innovations inspire us every day. To Our Lean, Mean DHL Dream Team: Mike Mand, JD, PhD – 9 DHL years: A powerhouse blend of legal acumen and deep biotech insight Vasanth Vaidyanathan, PhD – 4 DHL years: Inter
Apr 3, 2025


Protect Clinical Updates Before They're Disclosed
SUMMARY: Biologic candidates evolve during clinical trials - so should your patents. Updated filings before public trial disclosures (e.g., clinicaltrials.gov, conferences) can capture new, patentable refinements and avoid prior art issues. During clinical development of a biologic changes are often made in dosing, formulations, patient populations, and clinical endpoints. These refinements are frequently patentably distinct from initial filings. Beware: Clinical trial update
Mar 31, 2025


Is Your Biotech Patent Spend Too High?
Too many biotech companies treat IP like a checkbox. Smart ones prune ruthlessly—and build value, not just volume: Prune entire families that no longer support the pipeline. Cut spend in countries with low ROI. Re-align IP quarterly with product + clinical shifts. Read below to learn more. Patent spend can get out of control, unless you prune your company's low value patent families. This is especially true for biotech companies that are at least 3 years old. Pruning can be c
Mar 28, 2025


Is Your Patent Team Doing a Good Job in Building Your Patent Portfolio?
INTRODUCTION Executives usually ask these quantitative but high-level questions: How much money did we spend on patent work this year? Was that within our IP budget? How many patent applications did we file this year? How many patents issued to our company this year? While those questions touch upon important metrics, this post provides much deeper and more insightful questions to ask, to much more effectively assess your IP team’s performance at building your company’s paten
Mar 13, 2025


IP Diligence Checklist: Assure Your Company’s IP is in Order Before Reaching Out to Potential Investors or Acquirers
As a biotech company, much of your value is in (pre-revenue) and/or protected by (post-revenue) your intellectual property. As you attempt to approach investors for your next round of financing, or as you consider reaching out re: potentially being acquired, it is important that your intellectual property documents are in order, and you understand your IP strengths and weaknesses and strategy. Here's a diligence checklist annotated with some pro tips that you can use to assu
Feb 20, 2025


Key Considerations for Drafting Working Examples in Biotech Patent Applications
Working examples are an important part of a biotech patent application, especially because this is considered an unpredictable art in U.S. patent law. Thus, although not mandatory, working examples, which are often set out in a separate Examples section, usually help assure that claimed subject matter is enabled, described, and definite (i.e., meets the requirements of 35 USC 112 (a) and (b)). Furthermore, working examples can provide important data to help differentiate the
Feb 7, 2025


USPTO Patent Fee Increases Set for Jan 19, 2025, Probably
SUMMARY Costs for prosecuting patents at the USPTO are set to increase on 1/19/25 Across-the-board 7.5% increase in fees Some targeted fees increase more Some new fees added After-Final 2.0 program terminated as part of fee increase changes Still possible that someone will file a lawsuit to delay Implementation of the fee increases so the future Trump-appointed USPTO Director can reconsider them What new fees/increases are most relevant to biotech companies? Noteworthy is the
Dec 20, 2024
Get the latest DHL news and life science patent and investor insights right in your mailbox!
bottom of page
