top of page
Insights & News

Is Your Biotech Company Dedicated to Strong Patents?
The Lip Service Problem In 25+ years of working with biotech leadership teams, I’ve heard countless executives assure investors, employees, and boards: “We are committed to strong patents.” But too often, those words are just lip service. Even well-meaning leadership teams that believe they are prioritizing IP often aren’t. The difference between talk and true commitment comes down to one rarely asked question: “What percentage of your R&D budget is dedicated to experiments r
Sep 24, 2025


AI Hype vs. Reality: Lessons from PCs, the Internet, and Smartphones
As a professional whose career started in the 1980s, AI feels like the early days of PCs, the Internet, and smartphones—big hype, real potential, but real transformation takes time. Even if the tech moves fast, humans and systems take longer to adapt. ---------------- [FYI: The above text was generated by ChatGPT except that I added the 1st clause: "As a professional whose career started in the 1980s". (I left in the em dash (—) so you might have picked up that this was gener
Aug 20, 2025


Afraid that AI might take your job?
At DHL, we are committed to finding ways to use AI to help us do our patent tasks more efficiently and more effectively. We use AI every day to help us. But we realize that AI is in its early days and has many limitations. That's why we use it carefully and responsibly. About DHL Double Helix Law (DHL) has decades of experience building strong patent portfolios for life science companies. Learn more about DHL and meet the team . Want to stay updated on life science patent law
Aug 19, 2025


Trouble Getting Biotech Patents to Issue? Don't Blame the Examiner.
Why Your Biotech Patent Keeps Getting Rejected, and What to Do About It. If your biotech patent application keeps getting rejected, it’s tempting to blame the patent examiner. But in most cases, the real issue is a weak patenting strategy or poor execution — both of which can be fixed. Here’s how to diagnose what’s going wrong and turn things around. Point 1 – Are You Relying on Only One Examiner and One Claiming Strategy? If you have only one application, one examiner, and
Aug 10, 2025

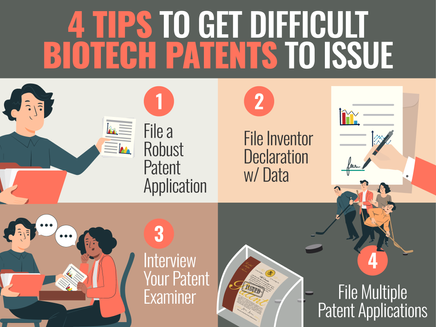
4 TIPS TO GET DIFFICULT BIOTECH PATENTS TO ISSUE IN THE U.S.
(Check out the short version of this post, if you don't want all the details in this patent professional version.) Introduction: It can often be difficult to get valuable patents covering biotech inventions to issue in the U.S. This is especially true if your patent team files quick and minimal patent applications and Office Action Responses, and uses a traditional reactive patent prosecution “strategy”. Alternatively, in this post, we provide 4 proactive and effective paten
Jul 8, 2025


KEEP YOUR ANNUAL IP SPEND WITHIN BUDGET WITH OUR PRIORITIZATION MATRIX TOOL (Full Version)
SUMMARY In this post we present a prioritization matrix tool that enables your IP team to dedicate an appropriate amount of resources to its highest value tasks and remain within its monthly, quarterly and annual budgets. For patent prosecution tasks, the matrix tool relies on prioritization not only at the patent family level, but at the individual patent application/country level as well. Such prioritization requires open and frequent communication with business, R&D, and
Jun 30, 2025


Assuring Your Patent And R&D Teams Are Well Connected
Biotech products and processes evolve over time. This can cause a misalignment between your issued/pending patent claims, and your improved processes and products. Thus, your patent team needs to stay closely connected to your R&D and product teams. Are your patent, product and R&D teams well connected? To find out, pose this question to your in-house IP coordinator/Chief patent counsel: Has an experienced patent attorney confirmed that the claims of our issued patents/pendin
May 7, 2025


Having Trouble Getting a Biotech Patent to Issue? Expert Declarations Can Clear the Way.
Biotech innovation is regularly faced with a thicket of prior art that applicants must navigate during patent prosecution. Examiners at the U.S. Patent and Trademark Office (USPTO) often combine various pieces of prior art to reject the claims as obvious. As we discuss in our recent article , arguments that can be made to overcome obviousness rejections, focus on the following with respect to the cited prior art: all the elements of the claims are not disclosed; a lack of a
Apr 20, 2025


Protect Clinical Updates Before They're Disclosed
SUMMARY: Biologic candidates evolve during clinical trials - so should your patents. Updated filings before public trial disclosures (e.g., clinicaltrials.gov, conferences) can capture new, patentable refinements and avoid prior art issues. During clinical development of a biologic changes are often made in dosing, formulations, patient populations, and clinical endpoints. These refinements are frequently patentably distinct from initial filings. Beware: Clinical trial update
Mar 31, 2025


Is Your Biotech Patent Spend Too High?
Too many biotech companies treat IP like a checkbox. Smart ones prune ruthlessly—and build value, not just volume: Prune entire families that no longer support the pipeline. Cut spend in countries with low ROI. Re-align IP quarterly with product + clinical shifts. Read below to learn more. Patent spend can get out of control, unless you prune your company's low value patent families. This is especially true for biotech companies that are at least 3 years old. Pruning can be c
Mar 28, 2025


Is Your Patent Team Doing a Good Job in Building Your Patent Portfolio?
INTRODUCTION Executives usually ask these quantitative but high-level questions: How much money did we spend on patent work this year? Was that within our IP budget? How many patent applications did we file this year? How many patents issued to our company this year? While those questions touch upon important metrics, this post provides much deeper and more insightful questions to ask, to much more effectively assess your IP team’s performance at building your company’s paten
Mar 13, 2025


IP Diligence Checklist: Assure Your Company’s IP is in Order Before Reaching Out to Potential Investors or Acquirers
As a biotech company, much of your value is in (pre-revenue) and/or protected by (post-revenue) your intellectual property. As you attempt to approach investors for your next round of financing, or as you consider reaching out re: potentially being acquired, it is important that your intellectual property documents are in order, and you understand your IP strengths and weaknesses and strategy. Here's a diligence checklist annotated with some pro tips that you can use to assu
Feb 20, 2025


Extended Patent Family Buildout - 3 Key Zones of Opportunity
INTRODUCTORY COMMENTS RE: BIOTECH/PHARMA EXTENDED PATENT FAMILIES In complex art areas like biotech/biopharma, patent strategy is about building extended patent families in key jurisdictions throughout the world, and especially in the U.S. This buildout should include numerous issued patents of different claim types and scope, from numerous related patent filings. These related filings typically share a lot of common disclosure, with numerous subsequent filings, typically pro
Aug 28, 2024


Annual Patent Planning Considerations
It's that time of year again... Annual patent planning for your company/client(s) for next year. Here are some key dates to track as you set your annual patent budget and help your team schedule their priorities: Regular application 12-month filing due dates for your priority applications (e.g. provisionals) filed this year. ⇨ Not only PCTs and regular U.S. app filing due dates, but consider non-PCT countries too. ⇨ For China, consider whether to directly file a utility and u
Dec 9, 2023
Get the latest DHL news and life science patent and investor insights right in your mailbox!
bottom of page
